News
Media and Press Releases
2023
- KitoTech Introduces 2-in-1 Wound Repair Kit to Consumers That Includes microMend® Wound Closure Devices and NuStat® Hemostatic Dressing to Stop Bleeding (Download PDF)
- KitoTech Medical Signs Agreement with Synergy Corporation for International Distribution of Their Medical and Consumer Products (Download PDF)
2022
- KitoTech’s microMend® Laceration Repair Kit Under Walgreens Private Label Brand Named Best New Store Brand Medical Product in 2022 (Download PDF)
- KitoTech Medical Signs Agreement with Corza Medical to Market microMend PRO Wound Closure Devices in U.S. (Download PDF)
2021
- KitoTech Medical Announces Series B Round Financing (Download PDF)
- KitoTech Medical Launches microMend® Skin Closure Variety Pack
- for Consumer Market (Download PDF)
- KitoTech Medical Exhibits at the American College of Emergency
- Physicians (ACEP) Scientific Assembly 2021 (Download PDF)
- KitoTech Medical Announces Dr. John Canady Has Joined as Its Chairman of Scientific Advisory Board (Download PDF)
- KitoTech Medical Announces David Krall Has Joined as Its Vice President of Sales and Marketing (Download PDF)
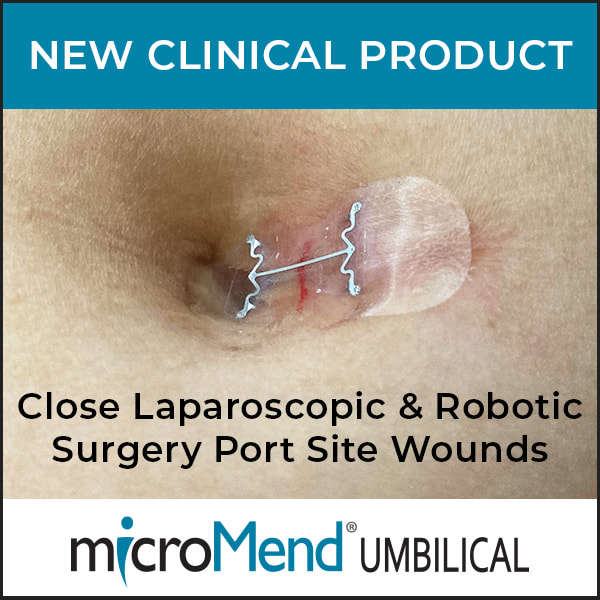
- KitoTech Medical Introduces microMend UMBILICAL Device for Closing the Umbilical Port Site in Laparoscopic and Robotic Surgeries (Download PDF)
- KitoTech Medical Introduces New microMend® Wound Closure Product for the Consumer Market (Download PDF)
- KitoTech Medical Announces New U.S. Patent Granted on Its microMend Wound Closure Products (Download PDF)
- KitoTech Medical Announces Completion of $4.5M Convertible Note Financing (Download PDF)
- KitoTech Medical Introduces microMend FLEX+™ for Closing Incisions Subject to Skin Stress and Movement in Orthopedics, Obstetrics and Other Specialties (Download PDF)
2020
- KitoTech Medical Awarded Non-Invasive Skin Closure Devices Agreement for microMend Products with Premier GPO (Download PDF)
- KitoTech Medical Announces Patrick Fry, a Leader in the Healthcare Industry, Has Joined Board of Directors (Download PDF)
- KitoTech Medical and MyMedic™ Announce Agreement to Include microMend Wound Closure Products into MyMedic’s First Aid Product Line (Download PDF)
- KitoTech Medical Issued Patent for Innovative microMend Wound Closure Products for Lacerations, Surgical Incisions and other Wounds
- KitoTech Completes $3M Convertible Note Financing to Expand the Use of its microMend Products for Medical and Consumer Markets (Download PDF)
- KitoTech Medical Introduces microMend WIDE for Rapid Closure of Long Incisions
- KitoTech Medical Launches shopmicromend.com as a Dedicated Consumer Site
- microMend Becomes Available on Amazon for Direct Consumer Purchase
- KitoTech Medical Introduces microMend Wound Closure Products for the Consumer Market
- KitoTech Medical Named One of 50 Leading Companies of The Year 2020 by The Silicon Review
2019
- KitoTech Medical Named One of the "Top Ten Medical Device Startup Companies for 2019" by MedTech Outlook
- KitoTech Medical Introduces Next Generation microMend SMALL at the Pediatric Emergency Medicine Assembly
2018
- microMend - a Novel Skin Closure Device
- KitoTech Medical Announces Agreement with North American Rescue
2017
- microMend Named One of the "Best Medical Technologies of 2017" by Medgadget
- microMend Skin Closure Device Introduced at ASDS in Chicago
- Bandage-Like Gadget Could Make Stitches and Staples a Thing of the Past
- This Brilliant ‘Skin Closure Device’ Could Replace Sutures and Staples for Millions of Medical Patients
- High-Tech microMend Bandage Could Replace Painful Stitches and Staples